|
April 2024 Mouse of the Month |
|
The relationship between CD103-positive DCs and oral tolerance C57BL/6-Tg(Itgae-HBEGF/EGFP)1Ksat (RBRC11949)
|
|
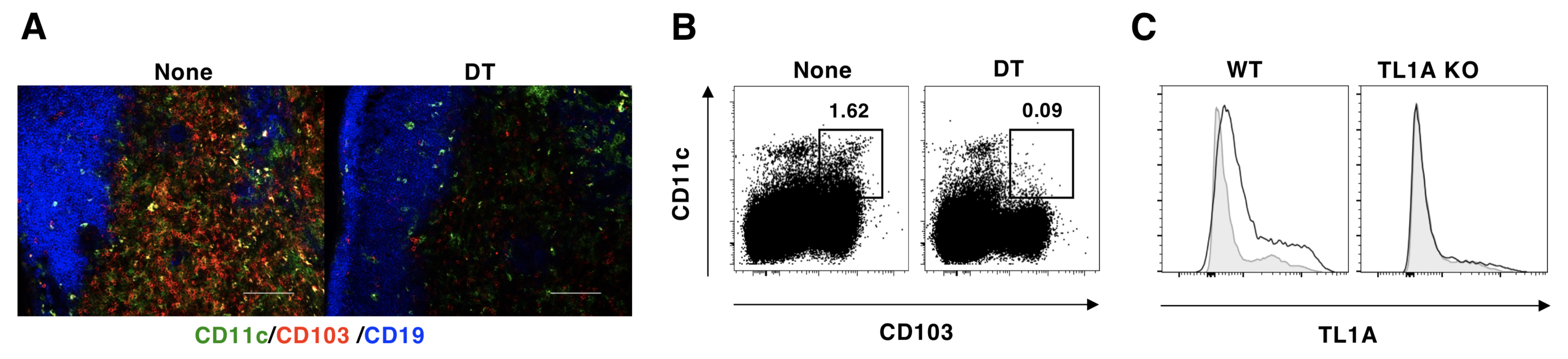
| In May 2023, the depositor (Dr. Katsuaki Sato) and his colleagues announced the mechanism that leads to the breakdown of oral tolerance by antibiotic-driven intestinal dysbiosis. This phenomenon leads to an increased risk of developing allergies. Therefore, the results of this research are attracting attention as a clue for the development of new treatments for allergies. We present two genetically modified mouse strains used in this research. B6.CD103-LSL-DTR mice (RBRC11949) are a BAC Tg strain with a STOP sequence flanked by loxP sequences (LSL cassette) and a human diphtherial toxin [DT] receptor (DTR)-EGFP cassette in the downstream of exon 31 of Cd103(Itgae) gene promoter region. CD103-positive cells express DTR in the presence of Cre recombinase, and administration of DT induces ablation of CD103-positive cells. CD103 is a cell adhesion molecule that belongs to the integrin superfamily. CD103 is known to interact with E-cadherin and localize immune cells to skin and mucosal tissues. Dr. Sato and his colleagues crossed B6.CD103-LSL-DTR mice with Cd11c gene Cre driver mice for conventional dendritic cells (cDCs) to generate CD103-positive cDCs-deficient mice and to analyze the function of CD103-positive cDCs. Tnfsf15 gene-deficient mice (RBRC11950) are deleted 3,014 bp including exons 2 and 3 of the Tnfsf15 gene by CRISPR/Cas9 system. Tnfsf15 gene belongs to the tumor necrosis factor (TNF) cytokine family and encodes TL1A (TNF-like ligand 1a). The receptor for TL1A is DR3 (TNFRSF25), a member of the TNF receptor family. TL1A is produced by cDCs and macrophages and acts on T cells and innate lymphocytes that express high levels of DR3 (TNFRSF25), which is known to be involved in inflammatory immune diseases. Dr. Sato and his colleagues clarified the relationship between decreased TL1A production in mesenteric lymph nodes and loss of tolerogenesis using this strain. Both B6.CD103-LSL-DTR mice and Tnfsf15 gene-deficient mice are useful strains that are expected to provide new insights into the field of immunology and related disease research. |
| Keywords | : | gut dysbiosis, oral tolerance, allergy, CD103, TL1A | |
| Depositor | : | Katsuaki Sato, Ph.D. (University of Miyazaki) | |
| Strain name | : | C57BL/6-Tg(Itgae-HBEGF/EGFP)1Ksat | |
| RBRC No. | : | RBRC11949 | |
| Strain name | : | C57BL/6-Tnfsf15<em1Ksat> | |
| RBRC No. | : | RBRC11950 | |
| Reference | : | [1] | Fukaya T, Uto T, Mitoma S, Takagi H, Nishikawa Y, Tominaga M, Choijookhuu N, Hishikawa Y, Sato K. Gut dysbiosis promotes the breakdown of oral tolerance mediated through dysfunction of mucosal dendritic cells. Cell Rep. 2023 May 30;42(5):112431. |
| April 2024 Saori Mizuno, Ph.D. Contact: Experimental Animal Division, RIKEN BioResource Research Center (animal.brc@riken.jp) All materials contained on this site may not be reproduced, distributed, displayed, published or broadcast without the prior permission of the owner of that content. |