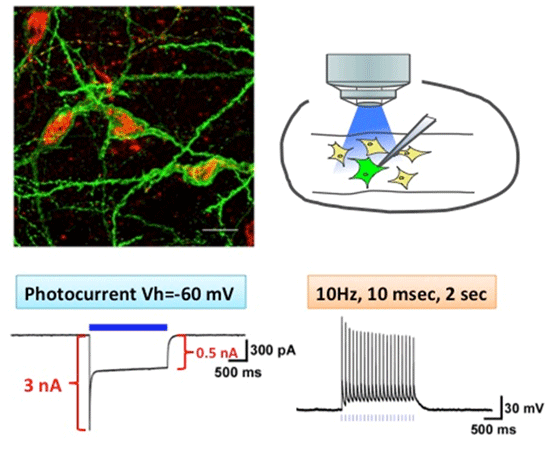
New reporter line for optogenetic stimulation at high frequenciesB6.Cg-Actb<tm1(tetO-ChR2*E123T/T159C)Ahky>/AhkyRbrc (RBRC05843)Courtesy of Akihiro Yamanaka, Ph.D. Channelrhodopsin-2(ET/TC) expression and function in melanin-concentrating hormone (MCH) neurons. B6.Cg-Actb<tm1(tetO-ChR2*E123T/T159C)Ahky> mice were bred with MCH-tTA mice (RBRC05844). The merged picture shows that ChR2(ET/TC) (green) is expressed in MCH-immunoreactive neurons (red). Slice patch clamp recordings from MCH neurons confirmed the function of ChR2. |
Optogenetic strategies for controlling neuronal activity have been widely employed for mapping functional circuits within the nervous system. Channelrhodopsin-1 (ChR1) and ChR2, related microbial-type rhodopsins from Chlamydomonas reinhardtii, are involved in the generation of photocurrents. When ChR2 is exogenously expressed in neurons, a flash of blue light evokes an inward current with membrane depolarization, generating an action potential [1, 2].
Several engineered ChRs have been designed to address limitations on precise optogenetic control. ChR2(C128S) was found to be more sensitive to light than wild-type ChR2, in the order of 102 or more. It has very slow deactivation kinetics and the photocurrent can be terminated with green light [3]. Prolonged activation of cells is possible with only brief illumination with blue light, which should minimize the toxicity caused by the illumination itself. Drs. Tanaka and Yamanaka exemplified the utility of the tet transgenic “KENGE-tet” system for optogenetic manipulation employing tetO-ChR2(C128S)-EYFP knockin mice (RBRC05454, see also Mouse of the Month, April 2013) [4]. To induce reliable and well-timed action potentials at high stimulation frequencies, Dr. Yamanaka established another mouse line that carries ChR2(E123T/T159C), a ChR2 variant that possesses two point mutations at positions E123 and T159. The recently reported E123T/T159C double mutant (ET/TC) enables reliable and sustained stimulation of neurons up to 40 Hz [5]. The tetO-ChR2(ET/TC) knockin mouse strain is a new powerful tool optimized for various experimental designs.
| Depositor | : | Akihiro Yamanaka, Ph.D. Research Institute of Environmental Medicine, Nagoya University |
|
| References | : | [1] | Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A.; 100(24):13940-5, 2003. |
| [2] | Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci.; 8(9):1263-8, 2005. | ||
| [3] | Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci.; 12(2):229-34, 2009. | ||
| [4] | Tanaka KF, Matsui K, Sasaki T, Sano H, Sugio S, Fan K, Hen R, Nakai J, Yanagawa Y, Hasuwa H, Okabe M, Deisseroth K, Ikenaka K, Yamanaka A. Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell Rep.; 2(2):397-406, 2012. | ||
| [5] | Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci U S A.; 108(18):7595-600, 2011. | ||