|
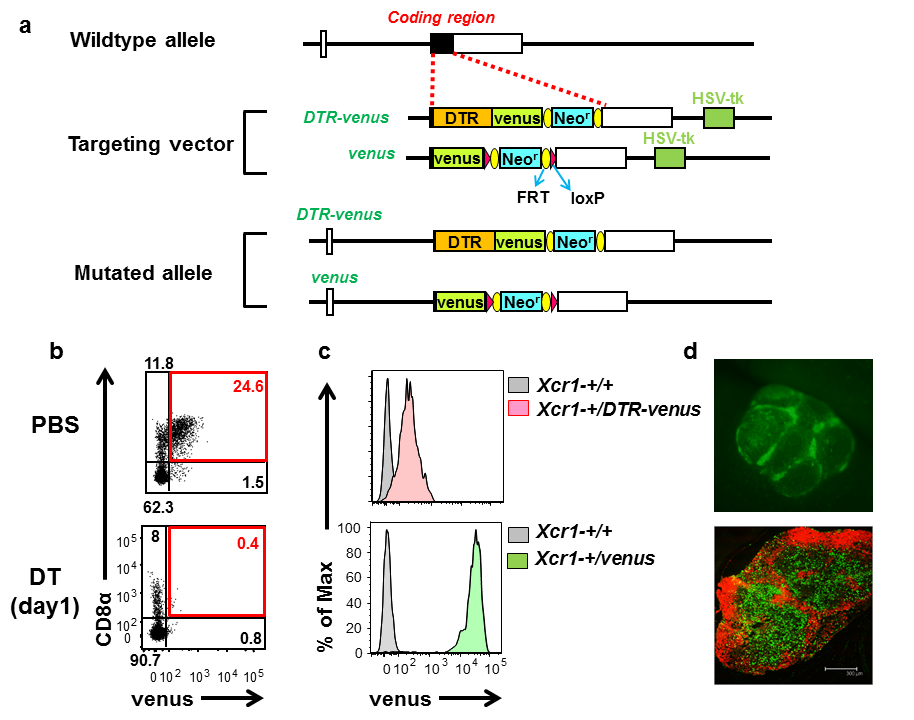
Tracking and depletion of XCR1-positive dendritic cells B6.Cg-Xcr1<tm2(HBEGF/Venus)Ksho> (XCR1-DTRvenus mice, RBRC09485)B6.Cg-Xcr1<tm1Ksho> (XCR1-venus, RBRC09486)Courtesy of Tsuneyasu Kaisho, M.D., Ph.D. (a) Schematic representation of the mouse wildtype Xcr1 allele, targeting vectors, and knocked-in alleles. Filled and open boxes denote coding and non-coding exons of Xcr1, respectively. DTR, Neor, and HSV-tk represent human diphtheria toxin receptor gene, neomycin-resistance gene, and herpes simplex virus thymidine kinase gene. (b) Venus expression and inducible depletion of XCR1+ DCs in XCR1-DTRvenus mice. Venus is expressed in murine CD8α+103+11b– DCs and the venus-expressing DCs are depleted one day after injection of diphtheria toxin. Numbers represent percentages of gated cells. (c) Venus expression levels of XCR1-DTRvenus and XCR1-venus mice. XCR1-venus mice show much higher expression levels of venus than XCR1-DTRvenus mice. (d) Venus expression (green) of XCR1-venus mice. A Peyer’s patch is observed by stereo-microscope (upper panel). A cryosection of an inguinal lymph node is stained with anti-B220 antibody (red) (lower). Venus expression can be detected without any anti-venus Abs. |
|
Dendritic cells (DCs) are professional antigen-presenting cells that play an important role in linking innate and adaptive immunity. DCs are heterogeneous and can be divided into several subsets, such as CD8α+103+11b– and CD8α–103–11b+ DCs, which are functionally distinct. DCs expressing a chemokine receptor, XCR1, are found in CD8α+103+11b– subset of DCs not only in the spleen but also in lymph nodes or peripheral tissues such as skin or intestine and show high ability to cross-present antigens on MHC class I molecules to initiate cytotoxic CD8+ T cell responses. Human XCR1 is also dominantly expressed in CD141+ DCs, which are featured by the crosspresenting ability and considered to be a human homologue of murine CD8α+103+11b– DCs. Therefore XCR1 is considered to be a marker for the crosspresenting DC subset in both mouse and human [1]. Kaisho and colleagues generated mouse strains to track and deplete XCR1+ DCs by knocking the genes encoding for a YFP derivative Venus or a fusion protein of human diphtheria toxin receptor (DTR) and venus into the Xcr1 locus [2]. XCR1+ DCs can be tracked by venus expression in both XCR1-DTRvenus (Xcr1-+/DTR-venus) and XCR1-venus (Xcr1-+/venus) mice. In terms of venus expression level, XCR1-venus mice are much more superior than XCR1-DTRvenus mice. In XCR1-DTRvenus mice, XCR1+ DCs can be transiently depleted by injection of diphtheria toxin, which cause cell death through human DTR. Thus, both XCR1-venus and XCR1-DTRvenus mice are useful tools to elucidate in vivo behavior and functions of XCR+ DCs in lymph nodes and peripheral tissues. |
| Depositor | : | Tsuneyasu Kaisho, M.D., Ph.D. (Laboratory for Host DTsuneyasu Kaisho, M.D., Ph.D. (Laboratory for Host Defense, RIKEN RCAI) Department of Immunology Institute of Advanced Medicine, Wakayama Medical University |
|
| Strain name | : | B6.Cg-Xcr1<tm2(HBEGF/Venus)Ksho> (XCR1-DTRvenus) | |
| RBRC No. | : | RBRC09485 | |
| Strain name | : | B6.Cg-Xcr1<tm1Ksho> (XCR1-venus) | |
| RBRC No. | : | RBRC09486 | |
| References | : | [1] | Yamazaki C, Miyamoto R, Hoshino K, Fukuda Y, Sasaki I, Saito M, Ishiguchi H, Yano T, Sugiyama T, Hemmi H, Tanaka T, Hamada E, Hirashima T, Amakawa R, Fukuhara S, Nomura S, Ito T, Kaisho T. Conservation of a chemokine system, XCR1 and its ligand, XCL1, between human and mice. Biochem Biophys Res Commun.; 397(4):756-61, 2010. |
| [2] | Yamazaki C, Sugiyama M, Ohta T, Hemmi H, Hamada E, Sasaki I, Fukuda Y, Yano T, Nobuoka M, Hirashima T, Iizuka A, Sato K, Tanaka T, Hoshino K, Kaisho T. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J Immunol.; 190(12):6071-82, 2013. | ||
| January 2016 Contact: Shinya Ayabe, Ph.D. Experimental Animal Division, RIKEN BioResource Center All materials contained on this site may not be reproduced, distributed, displayed, published or broadcast without the prior permission of the owner of that content. |