|
May 2021 Mouse of the Month |
|
Cas9 Tg mice to realize efficient genome editing mouse production
B6;B6D2-Tg(CAG-hCas9)2Saku (RBRC10984)
|
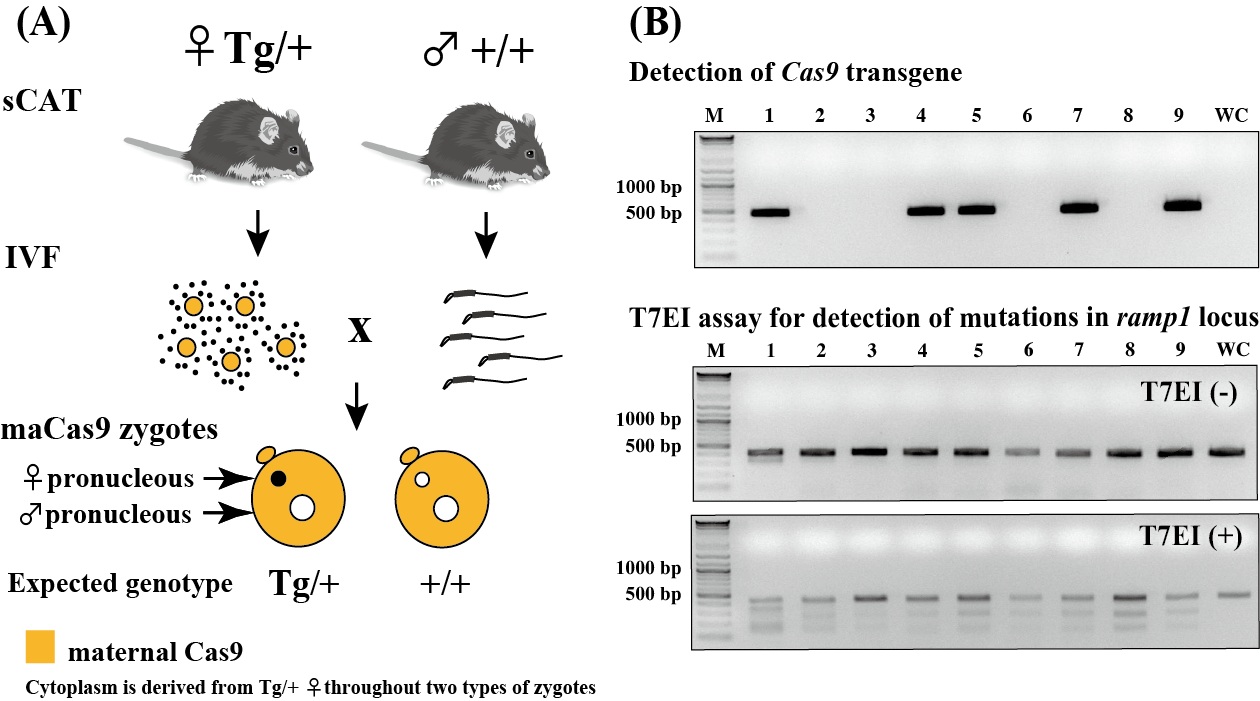
| Currently, the use of genome editing methods using the CRISPR / Cas9 system is becoming the mainstream for production of gene modified mice. However, there are still various problems that need to be resolved, such as low knock-in mutation efficiency for inserting foreign DNA into target site and difficulty of simultaneous mutations at multiple loci. sCAT (systematically Cas9-expressing transgenic) mice (RBRC10984) is a transgenic strain which expresses humanized Cas9 under the control of CAG promoter [1]. Depositor Dr. Sakurai and his colleagues developed a sCAT strain as a tool to solve the above problems and reported a novel genome editing mice production method; maCas9 (maternal Cas9)-based genome editing method. In this method, maCas9 zygotes (in vitro fertilization zygotes derived from sCAT oocytes) are used [2]. To examine the effect of maCas9 on knock-in mutations, they electroporated both arbitrary gRNA (guide RNA) for single locus and ssODN (single-strand oligo deoxynucleotide) as HDR (homology direct repair) knock-in template into maCas9 zygotes. As a result, compared with a conventional method which utilize exogenous Cas9 mRNA (or protein), low degree of mosaicism, higher knock-in mutation efficiency and increased pup delivery rate were shown. Moreover, to confirm the effect of maCas9 on simultaneous mutations at multiple loci, they electroporated 10 gRNAs into maCas9 zygotes and generated some multiple gene modified mice (a maximum of 9 loci). Compared with the conventional method with exogenous Cas9, higher indel (insertion/deletion) mutation efficiency and increased pup delivery rate were shown. From these results, maCas9-based genome editing method is useful for production of not only knock-in mice but also multiplex indel mutation mice. Therefore, sCAT mice will contribute to development of novel multifunctional disease mouse models in future. In addition, because sCAT mice express Cas9 mRNA in major organs (heart, testis, muscle, etc.), this strain will apply to the other in vivo or in vitro experiments. |
| Depositor | : | Takayuki Sakurai, Ph.D. Shinshu University |
|
| Strain name | : | B6;B6D2-Tg(CAG-hCas9)2Saku | |
| RBRC No. | : | RBRC10984 | |
| References | : | [1] | Sakurai T, Kamiyoshi A, Kawate H, Mori C, Watanabe S, Tanaka M, Uetake R, Sato M, Shindo T. A non-inheritable maternal Cas9-based multiple-gene editing system in mice. Sci Rep. 2016 Jan 28;6:20011. |
| [2] | Sakurai T, Kamiyoshi A, Kawate H, Watanabe S, Sato M, Shindo T. Production of genetically engineered mice with higher efficiency, lower mosaicism, and multiplexing capability using maternally expressed Cas9. Sci Rep. 2020 Jan 23;10(1):1091. |
||
| May 2021 Saori Mizuno, Ph.D. Contact: Experimental Animal Division, RIKEN BioResource Research Center (animal.brc@riken.jp) All materials contained on this site may not be reproduced, distributed, displayed, published or broadcast without the prior permission of the owner of that content. |