|
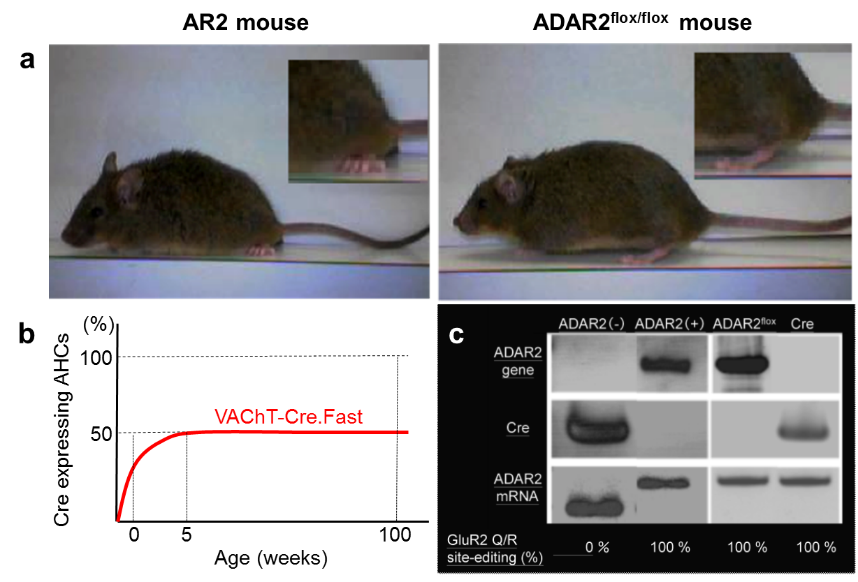
Pathomechanistic model mice of sporadic ALS: B6.Cg-Adarb1<tm1.1Skwa> Tg(SLC18A3-cre)KMisa (RBRC09428)Courtesy of Shin Kwak, M.D., Ph.D. (a) An AR2 mouse at 12 months of age (left panel) exhibits abnormal posture of the hind-limbs and the tail, as compared with normal posture of a control littermate (right panel). (b) Temporal profile of Cre expression in the motor neurons is indicated as the proportion of Cre-expressing and ADAR2-lacking motor neurons in the spinal anterior horns of the AR2 mice. Cre expression reached the maximum level (~50% of motor neurons) by postnatal week 5. AHCs: anterior horn cells. (c) The ADAR2flox gene, transcripts of the Cre gene and the ADAR2flox alleles before and after Cre-recombination in the laser-captured motor neurons were analyzed for each group by PCR. In addition, proportion of Q/R site-edited GluA2 in all the GluA2 expressed in motor neurons were calculated in ADAR2(−), ADAR2(+) motor neurons of AR2 mice and motor neurons of the littermate control mice (ADAR2flox/flox mice and VAChT-Cre.Fast mice). ADAR2 (−) motor neurons expressed only unedited GluR2 (GluA2) mRNA, harboring the truncated ADAR2floxgene and Cre transcripts, whereas ADAR2 (+) motor neurons expressed only edited GluR2 mRNA, carrying the full-length ADAR2flox gene and did not express Cre. Motor neurons of the littermate control mice expressed full-length ADAR2 and only edited GluA2 mRNA. |
| Amyotrophic lateral sclerosis (ALS) is a progressive adult-onset motor neuron disease. Patients with ALS develop progressive muscle weakness resulting from both upper and lower motor neurons and ultimately lose the ability to breathe without mechanical support within a few years of onset. Although little is known about pathogenesis of sporadic ALS, which accounts for more than 90% of ALS cases, recent studies demonstrated that failure of adenosine-inosine (A-I) conversion at the glutamine/arginine (Q/R) site of GluA2, a subunit of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, resulting from downregulation of an RNA-editing enzyme adenosine deaminase acting on RNA 2 (ADAR2) plays pivotal role in the ALS pathogenesis [1, 2]. AR2 mice mimic this molecular abnormality and exhibit ALS phenotype via mechanism mediated by Ca2+-permeable AMPA receptors that have Q/R site-unedited GluA2 in their subunit assembly [3]. In addition, TDP-43 pathology, a hallmark of ALS, is observed in the motor neurons devoid of ADAR2 immunoreactivity in the patients with ALS and ADAR2-lacking motor neurons of AR2 mice, in a calcium-dependent protease calpain-dependent manner [4, 5]. As restoration of ADAR2 activity by gene therapy using adeno-associated virus (AAV) rescues ALS phenotype and TDP-43 pathology in AR2 mice [6], the AR2 mice will be useful in ALS research aiming at both elucidating etiology and developing therapy. |
| Depositor | : | Keiji Itaka, M.D., Ph.D. & Shin Kwak, M.D., Ph.D. Laboratory of Clinical Biotechnology, Center for Disease Biology and Integrative Medicine Graduate School of Medicine, The University of Tokyo |
|
| Strain name | : | B6.Cg-Adarb1<tm1.1Skwa> Tg(SLC18A3-cre)KMisa | |
| RBRC No. | : | RBRC09428 | |
| References | : | [1] | Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature; 427(6977):801, 2004. |
| [2] | Hideyama T, Yamashita T, Aizawa H, Tsuji S, Kakita A, Takahashi H, Kwak S. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis.; 45(3):1121-8, 2012. | ||
| [3] | Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, Takahashi R, Misawa H, Kwak S. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci.; 30(36):11917-25, 2010. | ||
| [4] | Aizawa H, Sawada J, Hideyama T, Yamashita T, Katayama T, Hasebe N, KimuraT, Yahara O, Kwak S. TDP-43 pathology in sporadic ALS occurs in motor neurons lacking the RNA editing enzyme ADAR2. Acta Neuropathol.; 120(1):75-84, 2010. | ||
| [5] | Yamashita T, Hideyama T, Hachiga K, Teramoto S, Takano J, Iwata N, Saido T, Kwak S. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat Commun.; 3:1307, 2012. | ||
| [6] | Yamashita T, Chai HL, Teramoto S, Tsuji S, Shimazaki K, Muramatsu S, Kwak S. Rescue of amyotrophic lateral sclerosis phenotype in a mouse model by intravenous AAV9-ADAR2 delivery to motor neurons. EMBO Mol Med.; 5(11):1710-9, 2013. | ||
| November 2015 Contact: Shinya Ayabe, Ph.D. Experimental Animal Division, RIKEN BioResource Center All materials contained on this site may not be reproduced, distributed, displayed, published or broadcast without the prior permission of the owner of that content. |