|
BRC Current Technology January 2019 |
12. Boosting the efficiency of mouse cloning
|
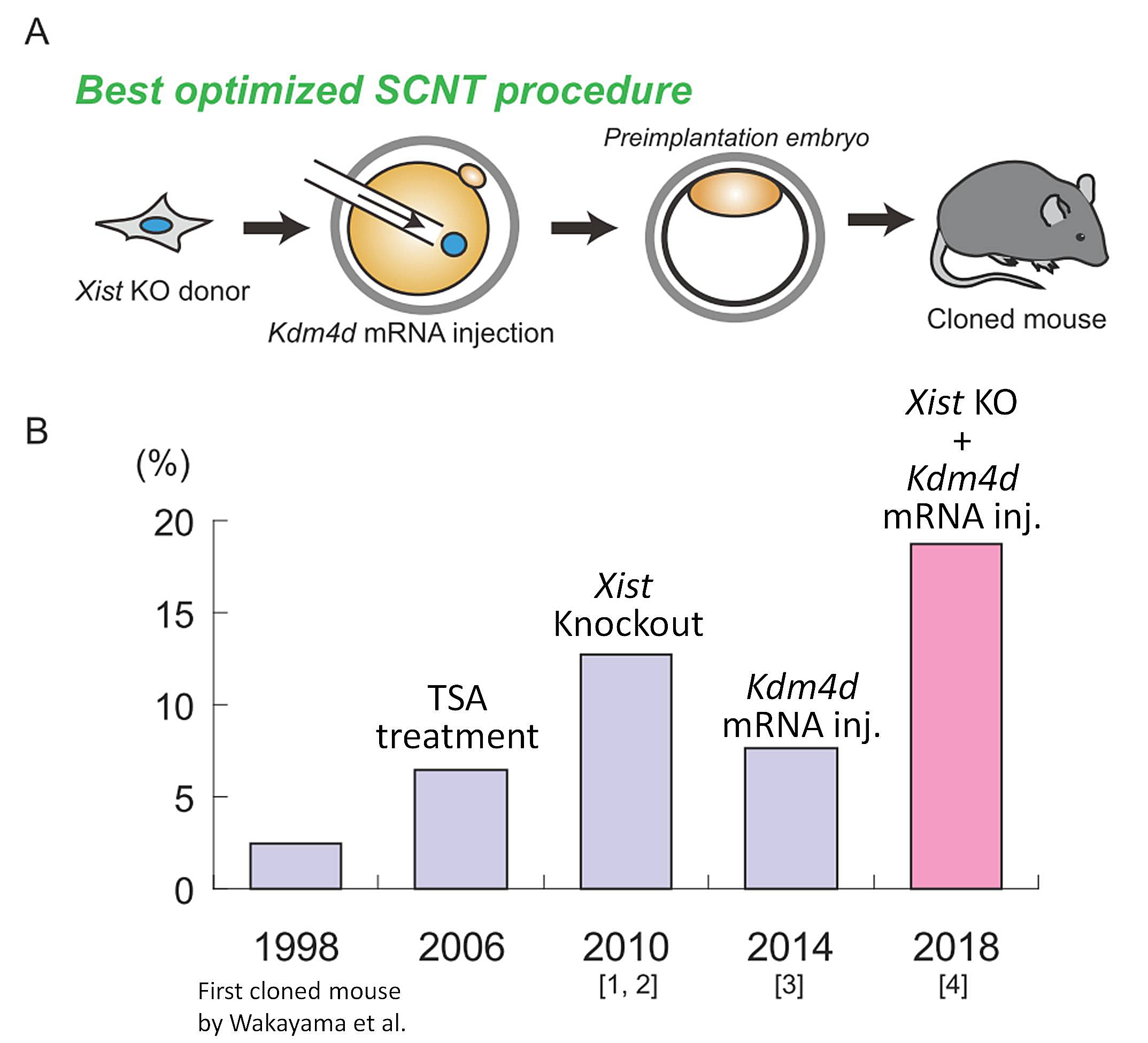
| RIKEN BRC has been working on technical improvements of somatic cell nuclear transfer (SCNT) technology. As SCNT enables cloning of animals, this technique allows us to expand mouse colonies with poor reproductive performance or with unique phenotypes. However, the extremely low success rate of cloning has made the practical use of this technique difficult. We have previously identified two critical epigenetic barriers, aberrant Xist activation and histone H3 lysine 9 trimethylation (H3K9me3). Aberrant Xist activation can be avoided by the use of Xist heterozygous knockout (KO) cells as nuclear donors [1, 2], and H3K9me3 can be removed by overexpressing a histone demethylase Kdm4d [3]. More recently, we have reported that a combined approach, concomitant use of Xist KO donor cells and Kdm4d mRNA, could improve the cloning efficiency to the highest level ever reported [4]. This best optimized mouse cloning method is expected to be applied to various mouse strains at RIKEN BRC. | |
| References | [1] | K. Inoue et al. “Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer.” Science, vol. 330, no. 6003, pp. 496–9, Oct. 2010. | [2] | S. Matoba et al. “RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos.” Proc. Natl. Acad. Sci. U. S. A., vol. 108, no. 51, pp. 20621–6, Dec. 2011. | [3] | S. Matoba, et al. “Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation.” Cell, vol. 159, no. 4, pp. 884–895, Oct. 2014. | [4] | S. Matoba, et al. “Loss of H3K27me3 Imprinting in Somatic Cell Nuclear Transfer Embryos Disrupts Post-Implantation Development.” Cell Stem Cell, vol. 23, no. 3, pp. 343–354.e5., 2018. |