1.Overview:
The lymphocytic choriomeningitis virus (LCMV) causes either acute or persistent infection in rodents, and opportunistic infection in humans. In order to detect LCMV infection in our mouse colony, Immunofluorescence assay (IFA) was carried out using LCMV infected cells against clinical serum samples collected from suspicious mice with LCMV infection.
2.Materials and methods
1) Preparation of LCMV infected cells
Vero E6 and L cells were infected with LCMV Armstrong and WE strains at the National Institute of Infectious Diseases (Tokyo, Japan) and Nagasaki University, (Nagasaki, Japan), respectively. The cells were trypsinized, washed with PBS, spotted on 14-well HT-coated slide glasses (AR Brown Co., Ltd., Tokyo, Japan), air dried, and fixed with acetone at room temperature for 5 min. The slides (IFA slides) were stored at -80°C until use. IFA was performed at Experimental Animal Division, RIKEN BRC.
2) IFA procedure
Ten μl of mouse sera (final dilution 1:20 and 1:100 by PBS) were applied on the IFA slides for 30 minutes at room temperature in a humidified chamber. After washing with PBS, FITC-conjugated goat anti-mouse immunoglobulin (H+L chain) antibody (SouthernBiotech, Birmingham, AL, USA) was used to detect primary antibodies. Slides were incubated in a dark humidified chamber for 30 minutes, washed with PBS, and covered using mounting medium (Vector Laboratories, Burlingame, CA, USA) and glass coverslips (Asahi Technoglass Corporation, Tokyo, Japan). Fluorescence microscope (Carl Zeiss Co., Ltd., Tokyo, Japan) was used for examination and photo recording. The cells were counterstained with appropriate dye such as Evans Blue or To-Pro3 (Molecular Probes, Invitrogen Corp., Carlsbad, CA, USA).
Mouse anti-LCMV Armstrong antiserum prepared at the National Institute of Infectious Diseases, Tokyo, Japan and mouse anti-LCMV monoclonal antibody (clone M104, American Research Products, Inc., Belmont, MA. USA) were used as positive controls.
3.Results:
The typical results are shown below (Figure 1). IFA revealed the existence of anti-LCMV antibodies in MAI/Pas and co-habited BALB/cA-nu/+ mice.
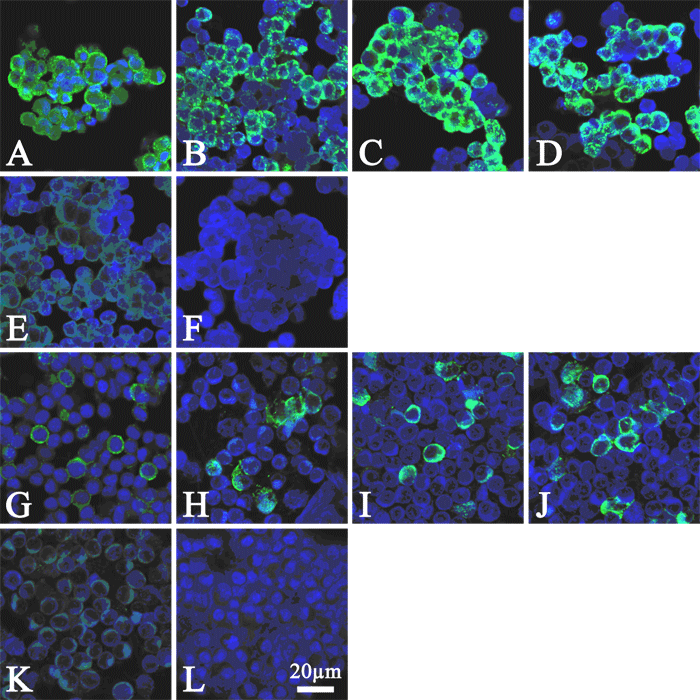
Figure 1.
Immunofluorescence assay (IFA) of antiserum for LCMV using Vero E6 cells (A-F) and L cells (G-L) infected with LCMV Armstrong and WE strains, respectively. Cells were counterstained with To-Pro3.
A, G: Test serum of infected MAI/Pas mouse. 1:20 dilution.
B, H: Test serum of a sentinel BALB/cA-nu/+ mouse caged with MAI/Pas. 1:20 dilution.
C, I: Mouse antiserum raised against LCMV Armstrong. 1:50 dilution.
D, J: Mouse monoclonal anti-LCMV (clone M104, culture supernatant, Acris Antibodies GmbH, Germany). 1:2 dilution.
E, K: Normal mouse serum of Jcl:ICR as negative control. 1:20 dilution.
F, L: Without the primary antibody. Negative control.
4.Conclusion:
IFA can detect anti-LCMV antibodies in the serum of a cohabited sentinel with a persistently-infected mouse.
Acknowledgements:
We acknowledge Professor Hiroshi Sato and Dr. Kazutaka Ohsawa, at Nagasaki University, Center for Frontier Life Sciences, Nagasaki, Japan, and Dr. Shigeru Morikawa at the Department Virology I, the National Institute of Infectious Diseases, Tokyo, Japan for IFA slides preparation. We also acknowledge Dr. Kazuhiro Takimoto at the Division of Experimental Animal Research, the National Institute of Infectious Diseases, Tokyo, Japan for the generous gift of mouse anti-LCMV antibody.
Fumio Ike, Ph.D.
Atsushi Yoshiki, Ph.D.
Experimental Animal Division
RIKEN BioResource Center




