
Multifunctional Rosa26 reporter mouse strain
B6;129S6-Gt(ROSA)26Sor<tm1(CAG-mTFP1)Imayo>/ImayoRbrc RBRC05146
Courtesy of Dr. Itaru Imayoshi, Kyoto University
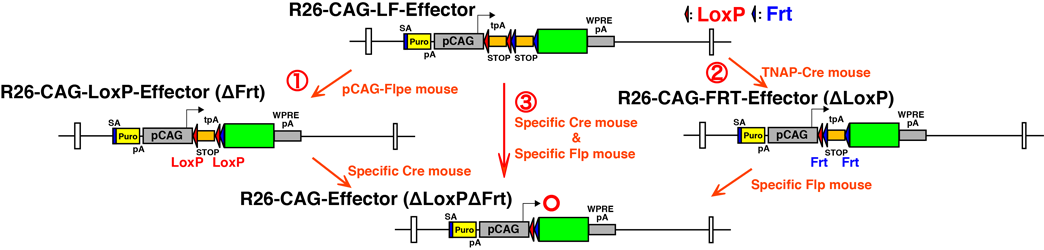
Schematic illustration of the Cre- and/or Flp-dual recombinase responsive alleles.
A transgene cassette composed of an SA (splice acceptor sequence)-puromycin resistance gene,
the CAG promoter, a floxed 3x SV40pA stop cassette (orange triangles), a 3x pA (SV40pA-TKpA-SV40pA)
stop cassette flanked by FRT sites (blue triangles), effector gene (green box), and WPRE-SV40pA was
knocked in at the Rosa26 locus. R26-CAG-LF-Effector was modified by the germline excision
of theFrt-flanked stop cassette using the pCAG-FLPe mouse to produce a Cre-dependent
effector line (R26-CAG-LoxP-Effector). The germline excision of the floxed stop cassette using
the TNAP-Cre mouse converts the R26-CAG-LF-Effector line to a Flp-dependent effector line (R26-CAG-FRT-Effector).
|
The Cre-loxP and Flp-FRT systems are very useful tools for time- and space-specific gene knockout [1]. Drs. Imayoshi and Kageyama generated multifunctional fluorescent reporter mice that strongly express monomeric teal fluorescent protein (mTFP1) after Cre- and/or Flp-mediated recombination [2]. mTFP1 is an improved variant of tetrameric cyan fluorescent protein (CFP) cFP484 from Clavularia coral. The reporter cassette was inserted into the Gt(ROSA)26Sor locus (R26-CAG-LF-mTFP1). The germline excision of the Frt-flanked stop cassette in R26-CAG-LFmTFP1 generates a Cre-dependent reporter (R26-CAG-LoxP-mTFP1). Similarly, R26-CAG-FRT-mTFP1, in which the loxP-flanked stop cassette is excised in the germline, requires only Flp to activate mTFP1 expression. These reporter mice are useful for monitoring Cre/Flp expression in tissues, and tracing the lineage of cells in embryos and adult mice at desired time points.
Drs. Imayoshi and Kageyama recently reported new mouse strains that express modifiedNatronomonas pharaonis halorhodopsin (eNpHR2)-EYFP fusion proteins after Cre- and/or Flp-mediated recombination in order to silence neural activity in vivo [3]. Optogenetic strategies for controlling neuronal activity have been widely employed for mapping functional circuits within the nervous system. These mouse strains also offer powerful tools for light-induced silencing of neural activity in genetically defined cell populations.
Fluorescent
reporter mouse
strains |
: |
Cre-&Flp-dependent mTFP1 mouse (R26-CAG-LF-mTFP1): RBRC05146
B6;129S6-Gt(ROSA)26Sor<tm1(CAG-mTFP1)Imayo>/ImayoRbrc
Cre-dependent mTFP1 mouse (R26-CAG-LoxP-mTFP1): RBRC05147
B6;129S6(B6)-Gt(ROSA)26Sor<tm1.1(CAG-mTFP1)Imayo>/ImayoRbrc
Flp-dependent mTFP1 mouse (R26-CAG-FRT-mTFP1): RBRC05148
B6;129S6(B6)-Gt(ROSA)26Sor<tm1.2(CAG-mTFP1)Imayo>/ImayoRbrc
mTFP1 mouse (R26-CAG-mTFP1): RBRC05149
B6;129S6(B6)-Gt(ROSA)26Sor<tm1.3(CAG-mTFP1)Imayo>/ImayoRbrc |
Knock-in mice
conditionally
expressing
halorhodopsin
eNpHR2.0 |
: |
R26-CAG-LF- eNpHR2-EYFP mouse: RBRC05150
B6;129S6-Gt(ROSA)26Sor<tm1(CAG-eNpHR2/EYFP)Imayo>/ImayoRbrc
R26-CAG-LoxP- eNpHR2-EYFP mouse: RBRC05151
B6;129S6-Gt(ROSA)26Sor<tm1.1(CAG-eNpHR2/EYFP)Imayo>/ImayoRbrc
R26-CAG-FRT- eNpHR2-EYFP mouse: RBRC05152
B6;129S6-Gt(ROSA)26Sor<tm1.2(CAG-eNpHR2/EYFP)Imayo>/ImayoRbrc |
| Depositor |
: |
Drs. Itaru Imayoshi and Ryoichiro Kageyama
Institute for Virus Research, Kyoto University |
| References |
: |
Mouse strains generated by Dr. Imayoshi (link to the depositorfs homepage)
http://imayoshi.web.fc2.com/Itaru_Imayoshi_Ph.D./Mouse_strains.html
| [1] |
Imayoshi I, Sakamoto M, Kageyama R. Genetic methods to identify and manipulate newly born neurons in the adult brain. Front Neurosci.; 5:64, 2011. |
| [2] |
Imayoshi I, Hirano K, Sakamoto M, Miyoshi G, Imura T, Kitano S, Miyachi H, Kageyama R. A multifunctional teal-fluorescent Rosa26 reporter mouse line for Cre- and Flp-mediated recombination. Neurosci Res.; 73(1):85-91, 2012. |
| [3] |
Imayoshi I, Tabuchi S, Hirano K, Sakamoto M, Kitano S, Miyachi H, Yamanaka A, Kageyama R. Light-induced silencing of neural activity in Rosa26 knock-in mice conditionally expressing the microbial halorhodopsin eNpHR2.0. Neurosci Res.; 75(1):53-8, 2013. |
|






