|
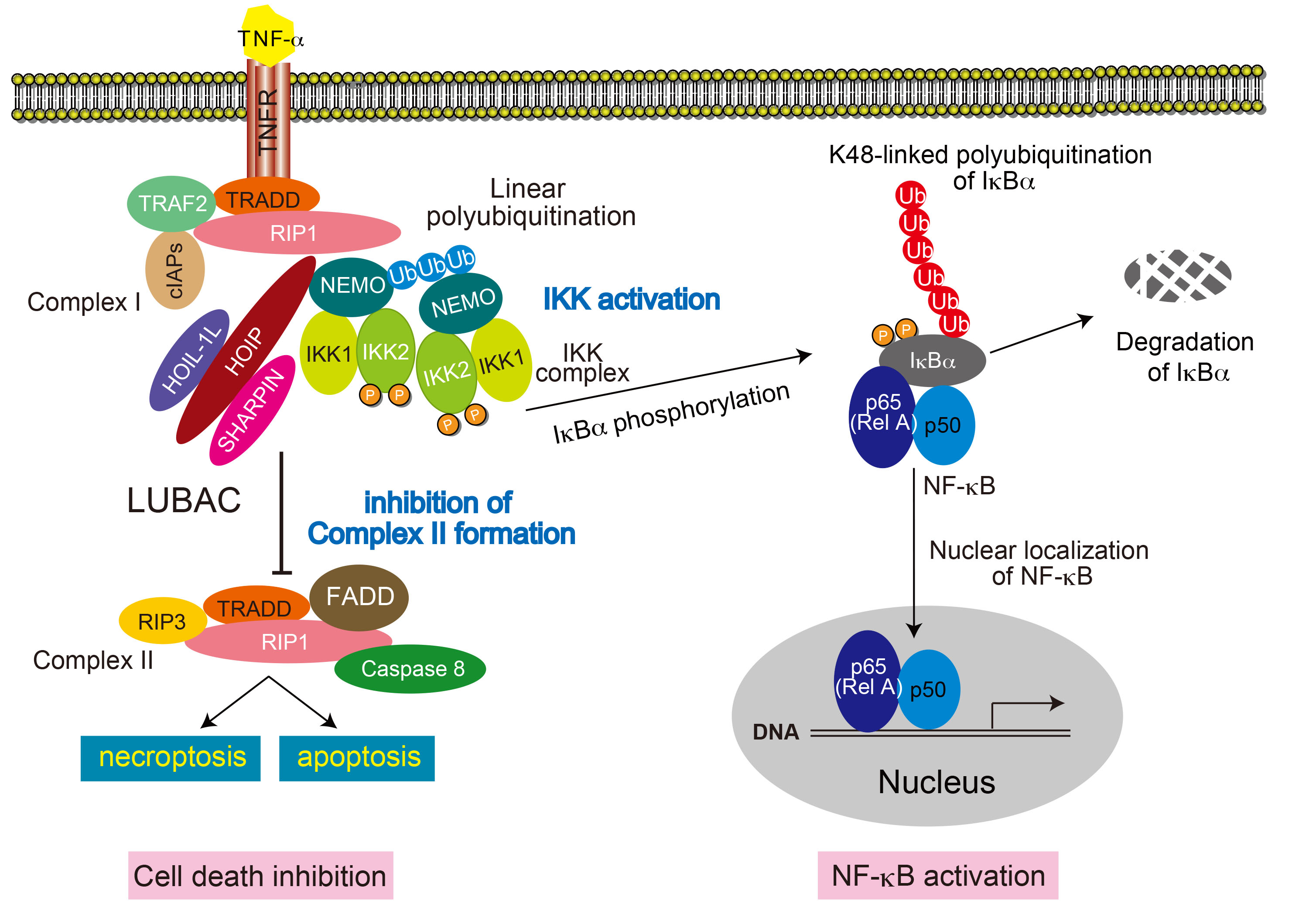
Activation of NF-κB pathway and suppression of cell death through linear polyubiquitination activity of LUBAC B6.B6CB-Rnf31<tm1.1Kiwa> (RBRC09483)Courtesy of Kazuhiro Iwai, M.D., Ph.D. Upon TNF-α stimulation, LUBAC conjugates linear polyubiquitin chains to NEMO. Linear polyubiquitination of NEMO activates the IKK complex. The activated IKK complex phosphorylates IκBs to activate the canonical NF-κB. LUBAC-mediated linear ubiquitination is also known to suppress TNF-α-mediated cell death by inhibiting formation of complex II although precise molecular mechanism remains unsolved. |
|
Ubiquitination is one of the post-translational modifications of cellular proteins. It regulates protein functions through conjugation of ubiquitin chains in most cases. Although polyubiquitin chains are mainly formed with one of the seven Lys residues from the ubiquitin molecule, linear ubiquitin chain assembly complex (LUBAC) specifically generates linear polyubiquitin chains via α-amino group of N-terminal Methionine of ubiquitin. LUBAC ubiquitin ligase is composed of two accessory subunits, heme-oxidized IRP2 ubiquitin ligase-1L (HOIL-1L) and SHANK-associated RH domain protein (SHARPIN), and the catalytic subunit, HOIL-1L–interacting protein (HOIP). The RING-IBR-RING (RBR) domain of HOIP is the catalytic center of linear polyubiquitination [1]. Canonical NF-κB pathway predominantly results in expression of cytokines and anti-apoptotic proteins via RelA activation. Recent studies have revealed that LUBAC promotes the assembly of linear ubiquitin chains on NEMO and activates the canonical NF-κB pathway and suppresses TNF-α-mediated cell death [1-4]. Mice lacking LUBAC ligase activity are embryonic lethal [1, 5]. Impaired B1 cell development and thymus–dependent and –independent type II immune responses were observed in mice with ablation of the catalytic domain of HOIP in B cells [5]. In addition to the canonical NF-κB, ERK activation pathways induced by CD40 and TACI were also impaired in B cells from these mice, showing that LUBAC-mediated linear polyubiquitination is essential for B-cell development and activation. The HOIP conditional knockout mouse strain, in which the catalytic RBR domain of HOIP is conditionally deleted, is a useful tool to clarify the role of polyubiquitination activity of LUBAC. |
| Depositor | : | Kazuhiro Iwai, M.D., Ph.D. Department of Molecular and Cellular Physiology Graduate School of Medicine, Kyoto University |
|
| Strain name | : | B6.B6CB-Rnf31<tm1.1Kiwa> | |
| RBRC No. | : | RBRC09483 | |
| References | : | [1] | Tokunaga F, Iwai K. Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.Endocr J.; 59(8):641-52, 2012./td> |
| [2] | Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation.Nat Cell Biol.; 11(2):123-32, 2009. | ||
| [3] | Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex.Nature; 471(7340):633-6, 2011. | ||
| [4] | Fujita H, Rahighi S, Akita M, Kato R, Sasaki Y, Wakatsuki S, Iwai K. Mechanism underlying IκB kinase activation mediated by the linear ubiquitin chain assembly complex.Mol Cell Biol.; 34(7):1322-35, 2014. | ||
| [5] | Sasaki Y, Sano S, Nakahara M, Murata S, Kometani K, Aiba Y, Sakamoto S, Watanabe Y, Tanaka K, Kurosaki T, Iwai K. Defective immune responses in mice lacking LUBAC-mediated linear ubiquitination in B cells.EMBO J.; 32(18):2463-76, 2013. | ||
| February 2016 Contact: Shinya Ayabe, Ph.D. Experimental Animal Division, RIKEN BioResource Center All materials contained on this site may not be reproduced, distributed, displayed, published or broadcast without the prior permission of the owner of that content. |