PD-1 deficiency and autoimmunityB6.129S2-Pdcd1tm1Hon/HonRbrc RBRC02142C.129S2-Pdcd1tm1Hon/HonRbrc (N20) RBRC02363NOD.129S2(B6)-Pdcd1tm1Hon/HonRbrc RBRC02361MRL.129S2(B6)-Pdcd1tm1HonHonRbrc RBRC02364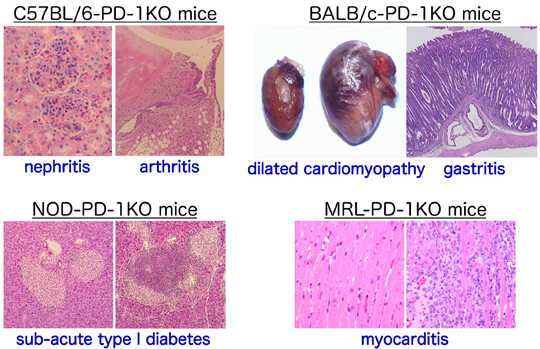 Pdcd1-knockout mice develop lupus-like glomerulonephritis and arthritis on the C57BL/6N background, autoimmune dilated cardiomyopathy and gastritis on the BALB/cCr background, sub-acute type 1 diabetes mellitus on the NOD/Shi background, and lethal myocarditis on the MRL background. |
T cell activation is controlled by a balance between the positive and negative signals that are transmitted between antigen-presenting cells and T cells. Programmed cell death-1 (PD-1, Pdcd1) is a co-receptor expressed in T cells that provides feedback inhibition. The ligands for PD-1, called PD-L1 (B7-H1) and PD-L2 (B7-DC), are expressed in B cells, T cells, macrophages, dendritic cells, and in a variety of tissues [1]. Studies on knockout mice have reported a significant role for PD-1 in the autoimmune response. Pdcd1-knockout mice develop lupus-like glomerulonephritis and arthritis on the C57BL/6N background [2], autoimmune dilated cardiomyopathy [3] and gastritis [4] on the BALB/cCr background, sub-acute type 1 diabetes mellitus on the NOD/Shi background [5], and lethal myocarditis on the MRL background [6]. It should be noted that the incidence of autoimmune dilated cardiomyopathy on the BALB/cCr background differs across generations of backcrossing. Approximately 30% of mice at N10 (RBRC00903) develop autoimmune dilated cardiomyopathy, while this is rarely observed in mice at N12 (RBRC00904) and N20 (RBRC02363). Mice at N20 are especially useful when autoimmune dilated cardiomyopathy should be avoided.
PD-1 also plays critical roles in the regulation of the immune response to cancer, allergy, and chronic viral infection. Indeed, large clinical trials have shown that antibodies blocking PD-1 or PD-L1 are likely to provide a new benchmark for antitumor activity in immunotherapy [7]. Pdcd1-deficient mice provide the opportunity to investigate the PD-1-mediated signaling pathway and to develop novel therapies against autoimmune diseases, cancer, infectious diseases, allergic diseases, and other common diseases.
| Related strains | : | C.129S2-Pdcd1tm1Hon/HonRbrc (N10) RBRC00903 C.129S2-Pdcd1tm1Hon/HonRbrc (N12) RBRC00904 C57BL/6-Tg(Actb-Pdcd1)BHon/HonRbrc RBRC02365 |
| Depositor | : | Dr. Tasuku Honjo Department of Immunology and Genomic Medicine, Kyoto University |
| References | : | [1]Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol; 19(7):813-24, 2007. [2]Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity; 11(2):141-51, 1999. [3]Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science; 291(5502):319-22, 2001. [4]Okazaki T, Otaka Y, Wang J, Hiai H, Takai T, Ravetch JV, Honjo T. Hydronephrosis associated with antiurothelial and antinuclear autoantibodies in BALB/c-Fcgr2b-/-Pdcd1-/- mice. J Exp Med; 202(12):1643-8, 2005. [5]Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A; 102(33):11823-8, 2005. [6]Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, Hiai H, Honjo T, Okazaki T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol; 22(6):443-52, 2010. [7]Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med; 366(26):2517-9, 2012. |





