Critical roles of TRAF6B6;129P2-Traf6tm1Jino/JinoRbrc RBRC04950C.129P2(B6)-Traf6tm1Jino/JinoRbrc RBRC05386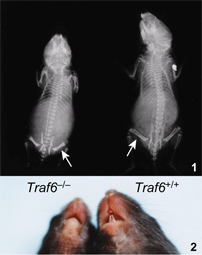 Among the live pups examined, only about 12% were Traf6-/-, while examination of the embryos revealed a normal Mendelian ratio of Traf6-/- mice at day 14.5 post-conception. Viable Traf6-/- mice appeared normal at birth, but became smaller than their normal littermates by day 6. Traf6-/- mice became more runted with time and died at 17-19 days. Whole body anteroposterior radiographs of 12-day-old Traf6-/-mice and their normal littermates revealed that the mutant mice had shortened long bones that were radio-opaque (Fig. 1) and one of their most obvious phenotypes was failure of tooth eruption (Fig. 2). These phenotypes are characteristic of osteopetrosis, a disorder of bone remodeling caused by impaired osteoclast formation or function. In fact, TRAF6 is required for signaling from RANK, an essential receptor for osteoclastogenesis. |
Tumor necrosis factor (TNF) receptor-associated factor (TRAF) is a cytoplasmic protein that is involved in signaling pathways via the TNF receptor superfamily and the Toll-like receptor/interleukin-1 (IL-1) receptor superfamily. TRAF6 activates the transcription factors NF-κB and AP-1, and plays essential roles in a variety of biological processes, including osteoclast differentiation, B cell follicle development, lymph node formation, central tolerance of T cells, development and maturation of dendritic cells, development of skin appendices, and survival of neuronal cells [1-14]. Therefore, loss of TRAF6 resulted in various disorders including osteopetrosis, hypohidrotic ectodermal dysplasia, autoimmunity, and a number of problems in both innate and acquired immune responses.
In the IL-1R and TLR pathways, IKK activation, an essential process for NF-κB activation, requires the generation of unanchored Lys63-linked polyubiquitin chains or their conjugation to TRAF6 and TGFβ-activated kinase (TAK) 1, both of which are catalyzed by TRAF6 (E3) and the Ubc13/Uev1A complex (E2). These Lys63-linked polyubiquitin chains act as platforms for the formation of active signal complexes that consist of MEKK3, TAK1, TAK1-binding (TAB) 2/TAB3, and the IKK complex. Formation of these complexes leads to the activation of TAK1, which then phosphorylates and activates IKKβ [15]. These knockout mice provide the opportunity to understand the role of TRAF6 in NF-κB activation in various biologically important systems.
| Depositor | : | Dr. Jun-ichiro Inoue Division of Cellular and Molecular Biology, The Institute of Medical Science The University of Tokyo |
| References | : | [1] | Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem; 271(46):28745-8, 1996. |
| [2] | Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells; 4(6):353-62, 1999. | ||
| [3] | Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J; 20(6):1271-80, 2001. | ||
| [4] | Naito A, Yoshida H, Nishioka E, Satoh M, Azuma S, Yamamoto T, Nishikawa S, Inoue J. TRAF6-deficient mice display hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A; 99(13):8766-71, 2002. | ||
| [5] | Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity; 17(6):823-33, 2002. | ||
| [6] | Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol; 173(5):2913-7, 2004. | ||
| [7] | Gohda J, Akiyama T, Koga T, Takayanagi H, Tanaka S, Inoue J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J; 24(4):790-9, 2005. | ||
| [8] | Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science; 308(5719):248-51, 2005. | ||
| [9] | Inoue J, Gohda J, Akiyama T. Characteristics and Biological Functions of TRAF6. In “TRAFs” edited by Hao Wu. Adv Exp Med Biol; 597:72-9, 2007. | ||
| [10] | Qin J, Konno H, Ohshima D, Yanai H, Motegi H, Shimo Y, Hirota F, Matsumoto M, Takaki S, Inoue J, Akiyama T. Developmental stage-dependentcollaboration between the TNF receptor-associated factor 6 and lymphotoxin pathways for B cell follicle organization in secondary lymphoid organs. J Immunol; 179(10):6799-807, 2007. | ||
| [11] | Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity; 29(3):423-37, 2008. | ||
| [12] | Konno H, Yamamoto T, Yamazaki K, Gohda J, Akiyama T, Semba K, Goto H, Kato A, Yujiri T, Imai T, Kawaguchi Y, Su B, Takeuchi O, Akira S, Tsunetsugu-Yokota Y, Inoue J. TRAF6 establishes innate immune responses by activating NF-kappaB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One; 4(5):e5674, 2009. | ||
| [13] | Motegi H, Shimo Y, Akiyama T, Inoue J. TRAF6 negatively regulates the Jak1-Erk pathway in interleukin-2 signaling. Genes Cells; 16(2):179-89, 2011. | ||
| [14] | Shimo Y, Yanai H, Ohshima D, Qin J, Motegi H, Maruyama Y, Hori S, Inoue J, Akiyama T. TRAF6 directs commitment to regulatory T cells in thymocytes. Genes Cells; 16(4):437-47, 2011. | ||
| [15] | Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal; 2(93):ra66, 2009. |





